A more fundamental definition of acids and bases than the one provided on the index page was given by the Danish chemists Johannes Broensted.
The definitions are:
ABroensted acidis a proton doner.
ABroensted baseis a proton acceptor.
The English chemist, Thomas Lowry, proposed exactly the same at roughly the same time so this is all called the Broensted Lewry theory.
The theory can be illustrated (figure 1)
Topic:Ideal Gas Law | Ionic Strength |
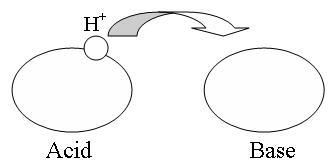
Fig 1.The proton is transferred from the acid to the base
So in terms of speaking about acids and bases in this context, a proton is H+. Please remember that hydrogen only consists of a proton and asingle electron giving the neutral charge. Without the electron only the proton is left, that is H+.
So, we can have both weak and strong acids. A weak acid is not very prone to release its proton while a strong acid will immediately donate its proton toanything that comes close.
In terms of definitions, a strong acid is fully ionized in aqueous solutions at normal concentrations. A weak acid is only partially ionized.
E.g. a solution of acetic acid held at pH 5.6, only approx. 10% is on the protonated form (CH3COOH) while 90% is ionized and in theform CH3COO–, having a negative charge since it has donated its proton H+to water (H2O) yielding the hydroniumion.
Topic:pKa& Ka | The basics |
Ions and compounds
Atoms are made up of neutrons that are neutral and have no charge, protons with a positive charge and electrons with a negative charge.
An ion is an entity or a group of atoms with a positive or negative charge such as the positively charged hydrogen ion H+and the negativelycharged nitrate ion NO3–. Negatively charged ions are also calledanionswhile positively charged ions are calledcations.
A compound is something that is made up of two or more simple substances. Ammonia NH3is a compound without a charge.
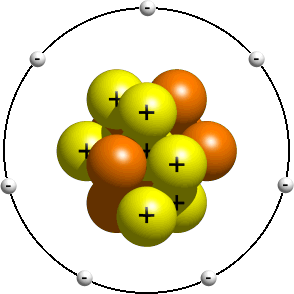
Atom model
In the diagram below, it is demonstrated how positively charged protons (yellow) and neutral neutrons constitutes a nucleus in the atom while the negativelycharges electrons orbits the nucleus. This is a very simple model of the atom. A more complicated model would include a nucleus surrounded by orbitalswith electrons in them orbiting the nucleus.
Topic:Equilibriums | The pH scale |
pH meter
Background
pH meters have been used since 1936 when they were invented by the Danish company Radiometer. In contrast to many other measuring devices pH metersdo not measure concentrations, rather they measure activities. To be more specific the pH meter does not measure the concentration of hydrogen ions.
pH meters are used to measure theactivity of hydrogen ionsin aqueous solutions:
pH = -Log10{H+} orpH = -Log10aH+.[H+]
Note:{H+} is the normal way of writing the activity of hydrogen ions but aH+.[H+] is also used often.
The activity of hydrogen ions is calculated into a pH value that is a measure of the acidity or alkalinity/basicity of the solution. Most pH meters havea digital display connected to the probe or electrode where both the pH and the temperature in the solution can be read.
Advanced pH meters even have conductivity meters build in measuring conductivity in mS/cm. Below is a couple of pictures showing different types of pHmeters:
Calibrating a pH meter
Calibrating a pH meter is done semi automatically with the help of the technician (or you) using the pH meter. Usually 2 or 3 solutions are usedfor the calibration procedure that involves telling the pH-meter that a calibration is going to take place and then measuring pH in different coloredaqueous buffer solution with pH values of 4, 7 and 10 or sometimes other pH values depending on pH meter type and manufacturer.
The software in the pH meter knows when the electrode is in the pH 4 solution and when the electrode is in the solution with a pH value of 10.
Other pH meter relevant information
Cleaning a pH meter:
pH meter should be cleaned regularly by applying a cleaning solution on the glass electrode
In between experiments:
The glass electrode should be submerged in a aqueous solution provided by the manufacturer; usually a solution with a pH of 3.
Considerations before buying a pH meter
If you are going to buy pH meters for your department you might as well consider buying at least one pH meter with a build in conductivity meter.
Also, you might choose a pH meter with a build in thermometer so you don’t have to type it into the pH meter yourself when you are going to calibrate.Secondly, and depending on what you are working with, you might also consider having a wire from the electronic pH meter to the probe of a length more than1 foot.
pH strips
Background
Substances with the property to change color when they come into contact with an either acidic or basic solution are called pH indicators. This is usedin pH strips that changes color when they come into contact with hydrogen ions (H+) and hydroxide ions (OH–).
Usually, pH strips are bought in packages with either 50 or 100 strips. pH strips are most useful for demonstration experiments or experiments whereaccuracy of the measured pH is of minor importance.
Using pH strips is simple. The strip is simply submerged in the aqueous solution and the reading of pH is done by comparing the color on the pH stripwith the color on the package that the pH strips came in.
The accuracy of any pH reading is within 0.5 pH units.
Web resources
A full course about atoms and ions
Concept explained
Broensted-Lowry at Salisbury University