The representatives of the world health organization said Thursday that China begin testing two drugs against the coronavirus. Early results may be available within three weeks.
One of the tests includes ramdevpir, an experimental antiviral drug made by Gilead. It is a remedy against coronavirus have not yet been licensed for use on a large scale.
The drug has been tested against the Ebola virus in the Congo, where he was not very effective. But when he gave the first American infected with the coronavirus, he recovered.
The second treatment that is tested, is the combination of two anti-H. I. V. drugs, ritonavir and lopinavir. This combination is sold as Kaletra in the United States and available in generic versions that are produced by several Indian pharmaceutical companies.
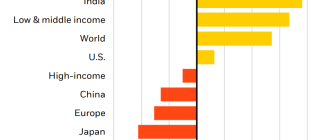
If any treatment helps prevent severe pneumonia, sepsis or organ failure in patients with coronavirus disease, mortality can be greatly reduced. Currently, according to the Chinese center for control and prevention of diseases, almost 5 percent of patients with coronavirus critically ill, and 2.3% die.
These mortality rates and severity based on the initial study of nearly 45,000 patients, China released the C. D. C. on Monday.