pH Scale
This site is about five different inorganic chemistry subjects related to pH.
Basic concepts – the pH scale
pH can be measured by a hydrogen sensitive electrode or a pH meter. pH is defined as the negative base 10 logarithm of the hydrogen ion activity{H+}, and not the hydrogen ion concentration [H+]:
pH = -Log10{H+}
| Topic: Ideal Gas Law
| Ionic Strength
|
There’s a consensus among chemistry teachers that students should be taught that pH = -Log10[H+] until they reach universitylevel where they are told (at least at chemistry departments) the truth that pH = -Log10{H+}.
If you read this and have been told that pH = -Log10[H+] please keep on reading. You will still be able to learn from the informationprovided here at pH scale.
In the section about ionic strength there are examples and discussion about how [H+] is calculated from {H+} and why it is importantto be able to do so in some situations.
Topic: Equilibriums 
| The pH scale
|
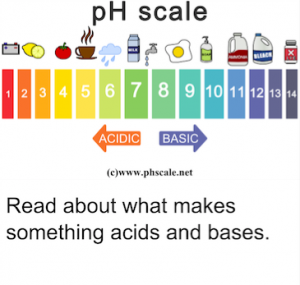
The pH scale
The range of the pH scale is from 0 to 14. The pH of a 1 mol/L HCl is solution is approximately 0 (because of ionic interactions between H2O,H+ and Cl–, the pH is not exactly 0 – see the ionic strength section). Any solution with a pH less than 7 is by definitionacidic.
Alkaline or basic solutions are those with a pH above 7. while neutral solutions have a pH of exactly 7.
Below is a picture of a scale showing pH from 0 to 14 with examples of solutions with different pH levels:
| Topic: pKa & Ka
| The basics |
Figure: The pH values of the shown solutions are approximate. E.g. the pH of seawater is usually from 7.5 to 8.4.
There are much more than discussions about the pH scale at this webpage, so please take your time to read or download the pdf files or read the webpages.
Ions and compounds
Atoms are made up of neutrons that are neutral and have no charge, protons with a positive charge andelectrons with… Read More
PDF library
Note:Discussions about the pdf documents are found in the 'Help & Discussions' section.Inorganic chemistry subjects:Equilibriums 1 :A tutorial about how… Read More
Acids and bases – Broensted-Lowry definition
A more fundamental definition of acids and bases than the one provided on the index page was given by the… Read More
Buffers and Equilibriums
As shortly explained in the section about how to calculate pH in a solution of dissolved NaHCO3, a buffer has… Read More
An introduction to Ionic Strength
BackgroundTo quantify the effect of inter-ionic interactions, such as molecular attraction and repelling, one has to have a `parameter` describing… Read More
Gases – the ideal gas law explained
Chemical compounds in aqueous solutions are fairly easy to handle as their quantities can either be expressed in weight such… Read More
pKa and Ka
Background informationThe Kavalue is a value used to describe the tendency of compounds or ions to dissociate. The Kavalue is… Read More
NaHCO3 dissolved in water – how is pH calculated?
Background about buffersA buffer is a solution that has the ability to keep pH within a certain and narrow range:… Read More
pH meter pH strips
BackgroundpH meters have been used since 1936 when they were invented by the Danish company Radiometer.In contrast to many other… Read More